Microblog
Breaking the Chain of Malarial Transmission: Progressing Vaccine Development Through the Characterization of Anti-Pfs25 Antibodies
 In 2018, there were 228 million cases of malaria leading to 405,000 deaths world-wide (World Malaria Report 2019) . With antimalarial drug resistance on the rise and billions of dollars being invested each year in malaria control and elimination efforts, the quest for an effective vaccination strategy remains a global priority. A major barrier to the development of a malaria vaccine is the complex life cycle of Plasmodium species. The parasites circulate between humans and mosquitos, with each stage exposing dramatically different antigens that could instigate an immune response. Transmission-blocking vaccines aim to elicit antibodies in a vaccinated human that, when ingested by a mosquito in a blood meal, prevent the parasites development in the mosquito, and subsequently block further transmission to another human (Wu et al. 2015) . McLeod et al. identify the most potent antibody to date against the Plasmodium surface protein Pfs25, and use molecular characterization to provide valuable insights to advance the development of a transmission-blocking vaccine (McLeod et al. 2019) .
In 2018, there were 228 million cases of malaria leading to 405,000 deaths world-wide (World Malaria Report 2019) . With antimalarial drug resistance on the rise and billions of dollars being invested each year in malaria control and elimination efforts, the quest for an effective vaccination strategy remains a global priority. A major barrier to the development of a malaria vaccine is the complex life cycle of Plasmodium species. The parasites circulate between humans and mosquitos, with each stage exposing dramatically different antigens that could instigate an immune response. Transmission-blocking vaccines aim to elicit antibodies in a vaccinated human that, when ingested by a mosquito in a blood meal, prevent the parasites development in the mosquito, and subsequently block further transmission to another human (Wu et al. 2015) . McLeod et al. identify the most potent antibody to date against the Plasmodium surface protein Pfs25, and use molecular characterization to provide valuable insights to advance the development of a transmission-blocking vaccine (McLeod et al. 2019) .
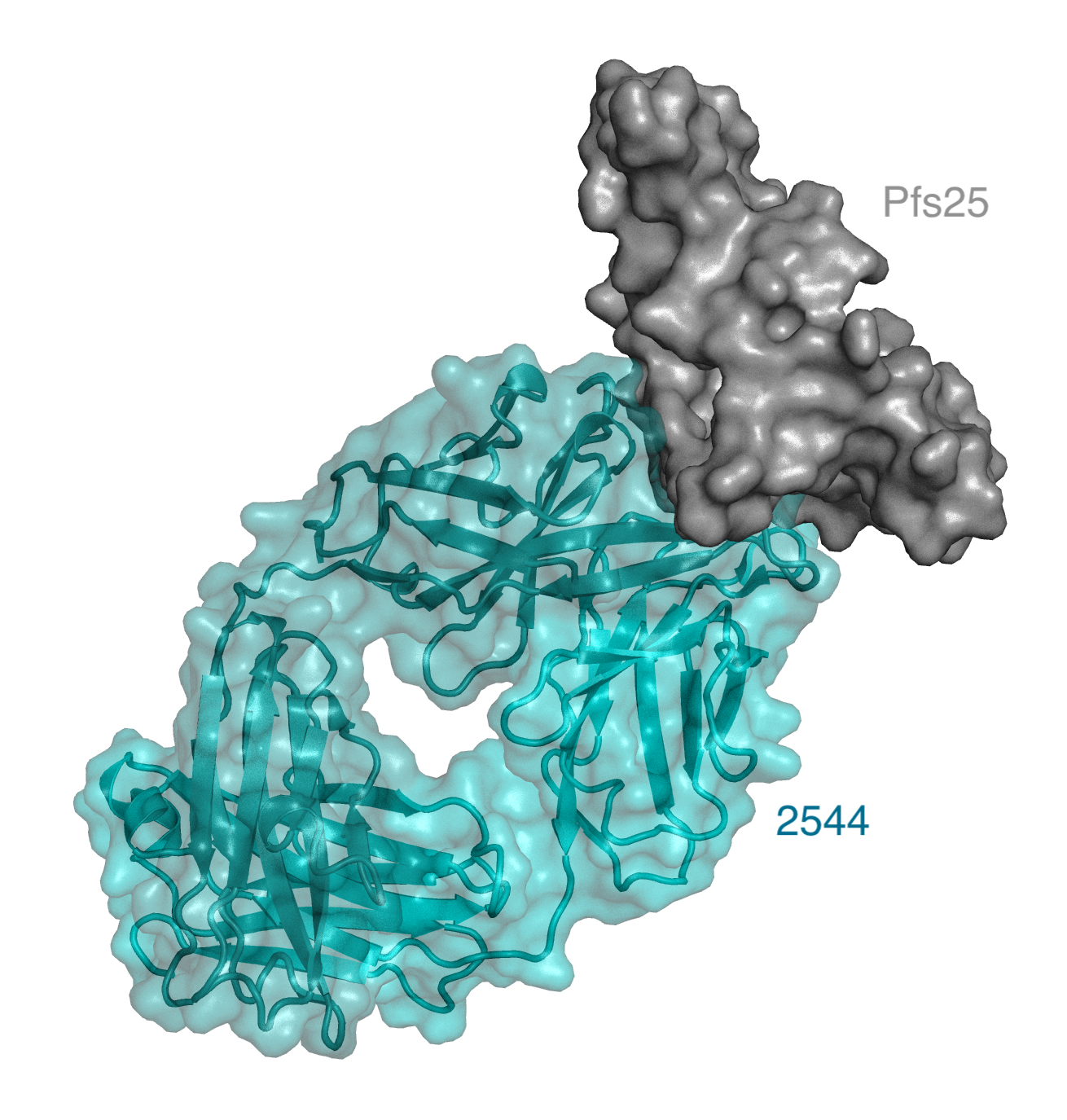
Pfs25 is a highly conserved protein important for Plasmodium development within the mosquito mid-gut. A challenge with targeting a mosquito-associated protein is mounting a strong enough immune response in humans that can be transferred to mosquitos during a blood meal (McLeod et al. 2019) . Researchers started with samples collected during a phase 1 clinical trial in which healthy volunteers were vaccinated with a formulation containing the Pfs25 protein (Chichester et al. 2018). From there, McLeod and colleagues were able to express 38 different antibodies produced by a single volunteer who mounted one of the strongest immune responses. Each antibody was tested for their binding affinity to the Pfs25 component of the vaccine, with eighteen binding directly to Pfs25. The eighteen antibodies were then triaged based on their ability to block parasite transmission is a mosquito feeding assay. In this assay, researchers tested the antibody inhibitory capacity by quantifying the parasitic abundance in mosquitoes eight days post infection. The researchers identified nine different anti-Pfs25 antibodies that resulted in over 80% reduction in parasite abundance, with one antibody, 2544, showing the highest transmission-blocking activity against Pfs25 ever reported.
Now that the researchers had established a set of effective Pfs25 binding antibodies, they aimed to characterize the location(s) on the Pfs25 protein that were recognized by the antibodies, termed the “epitopes”. By competing these new antibodies with those with known binding locations, and by obtaining crystal structures, the researchers established that most of the antibodies bound overlapping regions to two previously known sites (Scally et al. 2017) . Their findings also indicated that the two previously described sites were linked, serving as the immunogenic face of the Pfs25 protein. Interestingly, the researchers also identified a new site on Pfs25 recognized by only one of the antibodies. Furthermore, they demonstrated that there was no correlation between the location of antibody binding and the binding affinity.
McLeod et al. focused on the highest potency antibody, 2544, identifying sixteen amino acids that are present in the 2544 antibody but not in its antibody precursor, which was unable to bind to Pfs25. Guided by the crystal structures, they modeled the effect of individually reverting each amino acid to its precursor. From this analysis, they identified five amino acids as being critical for binding, and these alone were sufficient to increase the transmission blocking activity in the precursor to 78%.
Transmission-blocking vaccines are a truly altruistic approach to vaccine development; however, they have been shown to work synergistically with more conventional vaccination strategies that provide protection to the individual vaccinated. Therefore, using these approaches in combination could be a powerful tool in breaking the chain of malarial transmission, ultimately, leading to the eradication of this disease (Julien and Wardemann 2019) . By understanding the binding characteristics of potent antibodies such as 2544 to the Pfs25 protein, researchers can inform vaccine development. This tremendous work propels the development of a Pfs25 vaccine, supporting the viability of utilizing a transmission blocking vaccine to reduce the global burden of malaria infections.
Primary Research Article:
McLeod, B. et al. Potent antibody lineage against malaria transmission elicited by human vaccination with Pfs25. Nat. Commun. 10, (2019).
Other References:
Global Malaria Programme, W. G. World malaria report 2019. WHO (2019). Available at: https://www.who.int/publications-detail/world-malaria-report-2019. (Accessed: 19th April 2020)
Wu, Y., Sinden, R. E., Churcher, T. S., Tsuboi, T. & Yusibov, V. Development of Malaria Transmission-Blocking Vaccines: From Concept to Product. Adv. Parasitol. 89, 109–152 (2015).
Chichester, J. A. et al. Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: A Phase 1 dose-escalation study in healthy adults. Vaccine 36, 5865–5871 (2018).
Scally, S. W. et al. Molecular definition of multiple sites of antibody inhibition of malaria transmission-blocking vaccine antigen Pfs25. Nat. Commun. 8, 1–11 (2017).
Julien, J. P. & Wardemann, H. Antibodies against Plasmodium falciparum malaria at the molecular level. Nature Reviews Immunology 19, 761–775 (2019).