
Microblog
Killing Two Birds With One Stone: Preventing HIV Infection by Treating Parasitic Worms
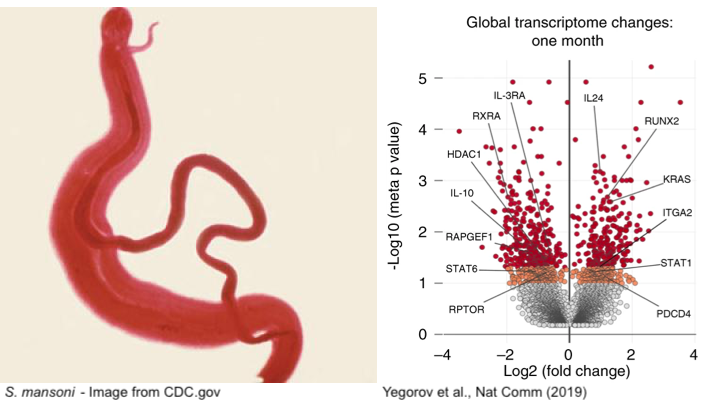 Human schistosomiasis is a neglected tropical disease caused by infection with parasitic flatworms contracted through exposure to contaminated water. Schistosomiasis affects over 200 million people worldwide, although, particular regions in Africa are disproportionately affected (WHO 2020). The helminth Schistosoma mansoni (Sm) causes intestinal schistosomiasis, whereby adult worms inhabit the gastrointestinal vasculature, producing eggs that are either excreted through feces or remain trapped in human tissues (McManus et al. 2018). Trapped eggs lead to inflammation-induced mucosal damage and changes in host immune defenses, which increases susceptibility to other pathogens (Colley et al. 2014). Therefore, it’s not surprising that observational studies have linked increased prevalence of HIV in women with schistosomiasis, with Sm-infected women being six times more likely to acquire HIV-1 (Downs et al. 2012). This association is hypothesized to be due to systemic immunological changes caused by schistosomiasis, suggesting that treatment of Sm may reduce susceptibility to genital HIV (Yegorov et al. 2018). To address this, Yegorov et al (2019) performed a prospective clinical study in a region in Uganda with a high occurrence of schistosomiasis.
Human schistosomiasis is a neglected tropical disease caused by infection with parasitic flatworms contracted through exposure to contaminated water. Schistosomiasis affects over 200 million people worldwide, although, particular regions in Africa are disproportionately affected (WHO 2020). The helminth Schistosoma mansoni (Sm) causes intestinal schistosomiasis, whereby adult worms inhabit the gastrointestinal vasculature, producing eggs that are either excreted through feces or remain trapped in human tissues (McManus et al. 2018). Trapped eggs lead to inflammation-induced mucosal damage and changes in host immune defenses, which increases susceptibility to other pathogens (Colley et al. 2014). Therefore, it’s not surprising that observational studies have linked increased prevalence of HIV in women with schistosomiasis, with Sm-infected women being six times more likely to acquire HIV-1 (Downs et al. 2012). This association is hypothesized to be due to systemic immunological changes caused by schistosomiasis, suggesting that treatment of Sm may reduce susceptibility to genital HIV (Yegorov et al. 2018). To address this, Yegorov et al (2019) performed a prospective clinical study in a region in Uganda with a high occurrence of schistosomiasis.
In this study, schistosome-infected HIV-negative adult women were enrolled from communities living in the Lake Victoria region of Uganda. At their initial visit, the participants were confirmed to be schistosome-positive and administered standard doses of praziquantel, a commonly prescribed drug for schistosomiasis treatment. At the one- and two-month follow-up visits, participants were monitored for successful schistosome treatment which was observed in about 90% of the participants. To evaluate whether Sm treatment affects susceptibility to HIV, Yegorov et al. performed ex-vivo HIV infection assays with the cervical and blood CD4+ T cells collected from study participants. In these assays, the collected cells were incubated with HIV pseudoviruses, and assessed for viral entry. The authors observed more than two-fold decrease in HIV entry into the cervical T cells at the one-month mark and this remained low at two months, providing evidence that treatment of Sm hinders HIV infection.
To further explore the cellular and molecular mechanisms behind this phenomenon, Yegorov et al. hypothesized that reduced viral entry is due to reductions in expression of the HIV co-receptor, CCR5, and/or impaired immune activation of CD4+ T cells. However, cervical CD4+ T cells isolated from participants post-Sm treatment displayed an increase in both CCR5 and the immune activation markers. Blood-derived CD4+ T cells did not show these changes, although the authors did note a systemic increase in the immune activation marker, CD69, at one month post-treatment. In accordance with these results, the authors observed elevated genital inflammatory cytokine levels, despite an overall decrease in blood cytokine levels. To explain the unanticipated observation of immune activation, the authors investigated interferon I (IFN-I) signaling. Global proinflammatory interferon I (IFN-I) levels are known to be stimulated by Sm egg antigens (Trottein et al. 2004), however IFN-I pathway activation also acts to downregulate Sm egg production in mice (Draz et al. 2010). Specific interferons were detected in genital and blood cells upon Sm treatment, once again demonstrating the compartmentalization of Sm-induced immune responses. Addition of the interferon detected in the genital tract, IFN-α2a, to CD4+ T cells from Sm-negative donors resulted in an upregulation in CCR5 and CD69 expression. Subsequent experiments found that these changes in co-receptor expression and immune activation prevented HIV entry in vitro, mirroring the in vivo findings.
Supporting their model, RNA-seq analyses performed on peripheral blood mononuclear cells (PMBCs) from Sm-infected participants revealed upregulation of genes associated with IFN-I signaling post Sm-treatment. The IFN and IFN-I regulated genes were differentially regulated one-month post-treatment, corroborating acute cellular and immunological changes associated with schistosomiasis treatment. On the other hand, Sm infection downregulated IFN-I signaling pathways, consistent with the model that Sm-treatment not only activates immune responses but also partially reverses Sm-dysregulated interferon signaling.
Overall, this study provides new evidence that treatment with praziquantel may lower HIV susceptibility for women infected with S. mansoni. Despite transient immune activation, schistosomiasis treatment significantly reduced HIV entry into cervical and blood CD4+ T cells for up to two-months. Furthermore, transcriptomic analysis on PMBCs identified interferon pathways and antiviral genes to be differentially regulated post-Sm treatment. These findings merit further investigation into schistosomiasis treatment as a potential strategy for safe and effective prevention of HIV in endemic regions.
Primary Reference:
Yegorov S, Joag V, Galiwango RM, Good SV, Mpendo J, Tannich E, et al. Schistosoma mansoni treatment reduces HIV entry into cervical CD4+ T cells and induces IFN-I pathways. Nature communications. 2019;10(1):2296.
Other references:
World Health Organization. Schistosomiasis Fact Sheet. http://www.who.int/schistosomiasis/epidemiology/table/en/ (2018). Accessed May 24, 2020.
McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat Rev Dis Primers. 2018;4(1):13.
Colley, D. G., Bustinduy, A. L., Secor, W. E., & King, C. H. Human schistosomiasis. Lancet. 2014; 383(9936), 2253–2264.
Downs JA, van Dam GJ, Changalucha JM, et al. Association of Schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg. 2012;87(5):868‐873.
Yegorov S, Galiwango RM, Good SV, et al. Schistosoma mansoni infection and socio-behavioural predictors of HIV risk: a cross-sectional study in women from Uganda. BMC Infect Dis. 2018;18(1):586.
Trottein F, Pavelka N, Vizzardelli C, et al. A type I IFN-dependent pathway induced by Schistosoma mansoni eggs in mouse myeloid dendritic cells generates an inflammatory signature. J Immunol. 2004;172(5):3011‐3017.
Draz HM, Mahmoud SS, Ashour E, Shaker YM, Wu CH, Wu GY. Effects of PEG-interferon-alpha-2A on Schistosoma mansoni infection in mice. J Parasitol. 2010;96(4):703‐708.

Frappier Lab
Posted on : 11/06/2020 9:00 AM
Liked this post?
Click the social media icons on the left to share!
Have anything to say? Comment below!



