
Microblog
Now You See Me: Discovery of a Light-Induced Secondary Metabolite from Hyphodiscus hymeniophilus Fungus
 Fungi represent a vast and largely untapped reservoir of secondary metabolites. With the ability to harm or heal, these compounds are being mined for antibacterial, antifungal, and antitumour activity (Keller, 2019). Despite the potential wealth of applications for fungal secondary metabolites, the fungal kingdom remains greatly underexplored, with only 120 000 species identified out of an estimated 2 million (Hawksworth and Lücking, 2017). Out of those identified, far fewer species have been thoroughly studied for their secondary metabolite production (Greco et al., 2019). In a quest to explore fungal secondary metabolites, Kramer et al. unearthed the natural product potential of an understudied filamentous fungus, Hyphodiscus hymeniophilus, and characterized its production of a novel, light-induced secondary metabolite.
Fungi represent a vast and largely untapped reservoir of secondary metabolites. With the ability to harm or heal, these compounds are being mined for antibacterial, antifungal, and antitumour activity (Keller, 2019). Despite the potential wealth of applications for fungal secondary metabolites, the fungal kingdom remains greatly underexplored, with only 120 000 species identified out of an estimated 2 million (Hawksworth and Lücking, 2017). Out of those identified, far fewer species have been thoroughly studied for their secondary metabolite production (Greco et al., 2019). In a quest to explore fungal secondary metabolites, Kramer et al. unearthed the natural product potential of an understudied filamentous fungus, Hyphodiscus hymeniophilus, and characterized its production of a novel, light-induced secondary metabolite.
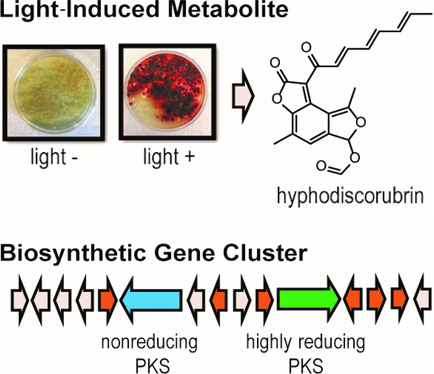
Kramer et al. performed a broad screen for fungal secondary metabolites with antibacterial, antifungal, and insecticidal activity. Although Hyphodiscus extracts did not possess microbicidal or insecticidal activity, the researchers’ attentions were captured by a reddish-brown metabolite produced by this fungus when grown in 12-hour light/dark cycles, but not under standard laboratory growth conditions. To uncover the metabolite responsible for this red pigmentation, the researchers analyzed the pigment via liquid chromatography-mass spectrometry and identified a single metabolite which they termed hyphodiscorubrin. During purification they observed that this metabolite was chemically reactive with amines – a common feature of molecules in the azaphilone metabolite class, which are often orange or red in colour. Using NMR, the researchers proposed a structure for hyphodiscorubrin, pictured above, which bore a structural resemblance to azaphilones. This diverse family of metabolites includes bioactive compounds such as the anti-inflammatory agent monascorubrin (Yasukawa et al., 1994), the food dye ankaflavin (Manchand et al., 1973), and the mycotoxin citrinin (Endo and Kuroda, 1976).
To uncover the natural product potential of H. hymeniophilus, Kramer et al. sequenced and assembled the first genome from this genus. Analysis of the fungal genome suggested that H. hymeniophilus can produce 24 distinct secondary metabolites from five different classes. Of these 24 distinct metabolites, 18 are potentially novel, with no homology to any characterized biosynthetic clusters. Further, the researchers identified a dual-polyketide synthase (PKS) cluster with homology to the azanigerone producing cluster from Aspergillus niger. Given that azanigerone is a member of the azaphilone metabolite class, the researchers hypothesized this biosynthetic cluster may give rise to hyphodiscorubrin. Reverse transcriptase-PCR amplification of one PKS cluster on RNA isolated from Hyphodiscus samples grown under both light and dark conditions showed that this cluster was transcribed at higher levels in the light, supporting the involvement of this cluster in the biosynthesis of hyphodiscorubrin. Kramer et al. explored the genome near the dual-PKS cluster to identify light regulatory mechanisms and found an adjacent retinal domain, which could play a role in regulating the light-induced expression of this cluster. Taking together the absence of antimicrobial or insecticidal activity of hyphodiscorubrin, and its light-induced expression, the authors concluded that the role of this compound is likely to protect the fungus from sunlight.
Overall, Kramer et al. provide the first genome-level exploration into the secondary metabolite potential of the Hyphodiscus fungal genus and discover a novel, light-induced metabolite, hyphodiscorubrin. The researchers demonstrate that the natural product potential of filamentous fungi can be much greater than the bioactivity captured during analysis of extracts grown in standard laboratory conditions. Altogether, this study suggests that new strategies should be applied to capture the diverse and valuable chemical potential of fungi for broad applications.

Primary Research Article and Image Citation: Kramer, G.J., Pimentel‐Elardo, S., and Nodwell, J.R. (2020). Dual‐PKS cluster for biosynthesis of a light‐induced secondary metabolite found from genome sequencing of Hyphodiscus hymeniophilus fungus. ChemBioChem cbic.201900689.
Other References
Endo, A., and Kuroda, M. (1976). Citrinin, an inhibitor of cholesterol synthesis. J. Antibiot. 29, 841–843.
Greco, C., Keller, N.P., and Rokas, A. (2019). Unearthing fungal chemodiversity and prospects for drug discovery. Curr. Opin. Microbiol. 51, 22–29.
Hawksworth, D.L., and Lücking, R. (2017). Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 5, FUNK-0052-2016.
Keller, N.P. (2019). Fungal secondary metabolism: regulation, function and drug discovery. Nat. Rev. Microbiol. 17, 167–180.
Manchand, P.S., Whalley, W.B., and Chen, F.C. (1973). Isolation and structure of ankaflavin: A new pigment from Monascus anka. Phytochemistry 12, 2531–2532.
Yasukawa, K., Takahashi, M., Natori, S., Kawai -i., K., Yamazaki, M., Takeuchi, M., and Takido, M. (1994). Azaphilones inhibit tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mice. Oncology 51, 108–112.




