
Microblog
Crohn’s and NOD2: A Classic Interaction Re-Examined
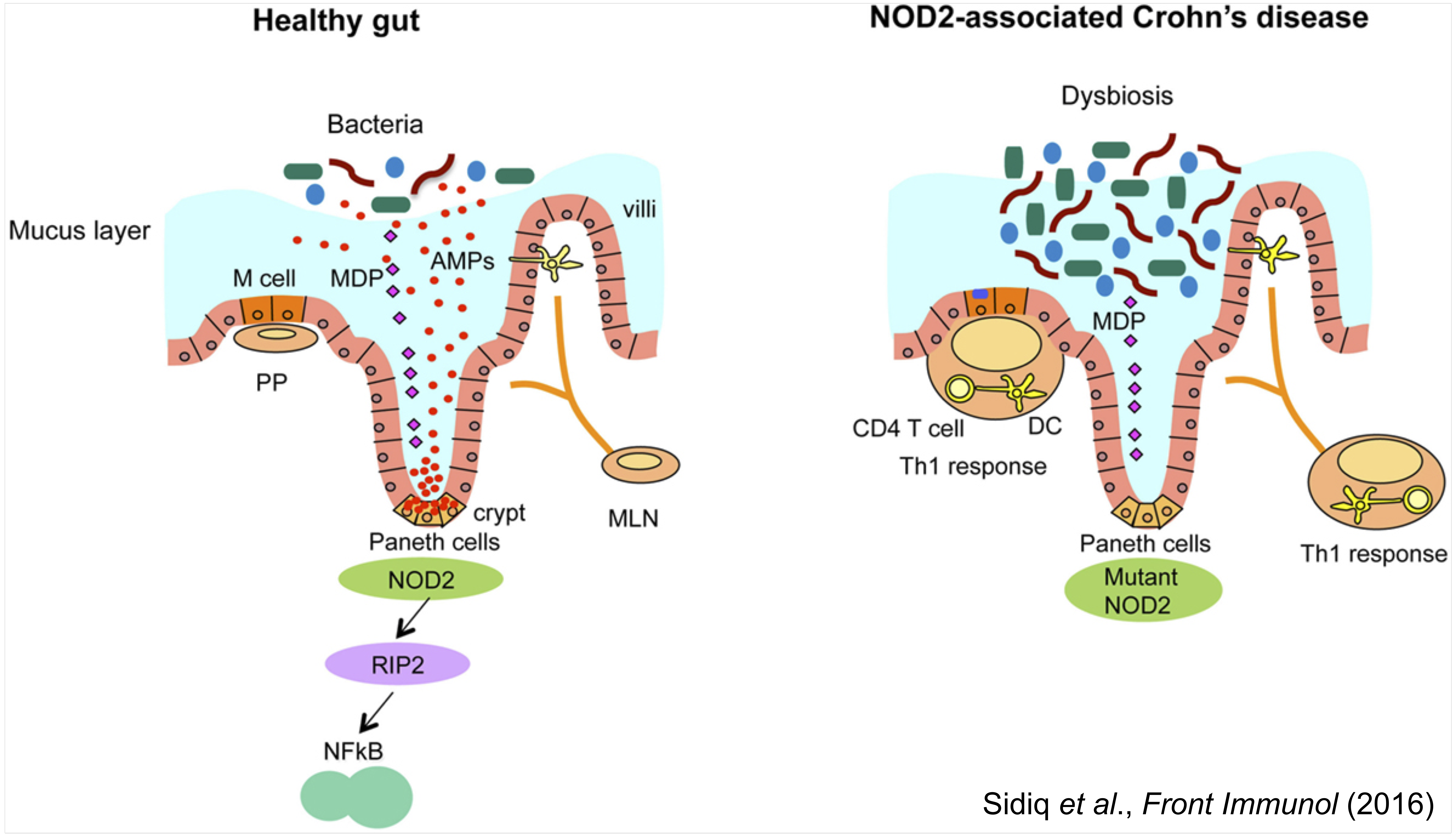 Canada has among the highest reported prevalence and incidence rates of inflammatory bowel disease (IBD) in the world, with 270,000 Canadians currently living with IBD. Crohn’s disease, an IBD characterized by severe gastrointestinal (GI) inflammation, inflicts 135,000 individuals across Canada and is associated with increased risk of colorectal cancer and overall mortality (Crohn’s and Colitis Canada). Despite its prevalence, we have limited understanding of the pathophysiology underlying Crohn’s and there is currently no cure. While the exact cause of Crohn’s is unknown, it is well-established that risk of disease is strongly correlated with having genetic mutations in NOD2, an intracellular sensor of the peptidoglycan-derived bacterial cell wall component, muramyl dipeptide (MDP) (Ogura, et al., 2001). However, until recently the connection between the loss of proinflammatory signalling in NOD2 mutants and the development of severe GI inflammation remained unclear. In a recent PNAS publication, Prescott et al. (2020) explore the immunomodulatory role of NOD2 and uncover fascinating connections with other factors implicated in aberrant GI inflammation, providing important contributions to the field that will help advance us towards finding a cure.
Canada has among the highest reported prevalence and incidence rates of inflammatory bowel disease (IBD) in the world, with 270,000 Canadians currently living with IBD. Crohn’s disease, an IBD characterized by severe gastrointestinal (GI) inflammation, inflicts 135,000 individuals across Canada and is associated with increased risk of colorectal cancer and overall mortality (Crohn’s and Colitis Canada). Despite its prevalence, we have limited understanding of the pathophysiology underlying Crohn’s and there is currently no cure. While the exact cause of Crohn’s is unknown, it is well-established that risk of disease is strongly correlated with having genetic mutations in NOD2, an intracellular sensor of the peptidoglycan-derived bacterial cell wall component, muramyl dipeptide (MDP) (Ogura, et al., 2001). However, until recently the connection between the loss of proinflammatory signalling in NOD2 mutants and the development of severe GI inflammation remained unclear. In a recent PNAS publication, Prescott et al. (2020) explore the immunomodulatory role of NOD2 and uncover fascinating connections with other factors implicated in aberrant GI inflammation, providing important contributions to the field that will help advance us towards finding a cure.
To re-examine the role of NOD2 in immunomodulation, the researchers began by monitoring the consequences of systemic activation of NOD2 via injection of MDP in mice. This led to a modest induction of regulatory T cells (Tregs) within the spleen, consistent with previous studies that have implicated Tregs in MDP immune responses (Leclerc, Bourgeois, & Chedid, 1982; Kishimoto et al., 1979). Dendritic cells (DCs) expressing the integrin CD103 are known to play a role in the induction of Treg responses (Coombes et al., 2007; Sun et al., 2007), and CD103 was also strongly upregulated on DC’s within the spleen upon administration of MDP. Upon further investigation, the authors found that CD103 was upregulated specifically on the cDC1 subset of DCs expressing CD24, XCR1, CD24, CD205 and CD8α, and not cDC2s, expressing CD11b and SIRPα (Collin & Bigley, 2018). This was an interesting finding as cDC1s expressing CD103 are critical for the development of immune self- tolerance, thus further prompting the authors to investigate the altered cDC phenotype (Qiu, et al., 2009).
To determine whether DCs themselves play a role in this immunosuppressive phenotype, the authors utilized a Cre-lox system to generate DC specific Nod2 knockout mice. Surprisingly, they found no difference in CD103 levels between the Nod2 knockout mice and their littermate controls upon MDP injection, indicating that this upregulation is not due to MDP recognition from DCs themselves. To proceed, the authors performed bone marrow chimera experiments wherein bone marrow from Nod2-sufficient mice was transferred into lethally irradiated Nod2-knockout mice, and vice versa, prior to MDP or vehicle injection. Interestingly, only Nod2-sufficient mice receiving Nod2-knockout bone marrow showed significant upregulation of CD103 on cDC1s upon MDP injection, indicating that this upregulation is reliant on NOD2 activity in non-hematopoietic cells.
Given this finding, the authors set out to find the intermediate messenger responsible for passing this signal from the non-hematopoietic cells to the DCs. The cytokine GM-CSF was their primary candidate, as it has been previously shown to modulate CD103 levels on cDC1s (Zhan et al., 2011). Indeed, intraperitoneal injection of GM-CSF produced a strikingly similar cDC1 phenotype to NOD2 activation via MDP. Furthermore, GM-CSF knockout mice failed to upregulate CD103 upon MDP injection, demonstrating NOD2 and GM-CSF indeed act in tandem.
To determine whether this phenomenon is relevant to Crohn’s disease, the researchers switched their focus to the intestine. They found that in the lamina propria (LP), the mucosal tissue that lines GI tract, CD103 expression was almost entirely restricted to cDC1, and this expression was significantly reduced in GM-CSF knockout mice. To further examine this phenotype in a more physiologically relevant manner, the authors induced a transient breach in the intestinal barrier via rectal administration of 50% ethanol and administered purified exogenous MDP to modulate intestinal DC populations. They assessed the effects of peptidoglycan sensing in DC populations following barrier breach in mice deficient in NOD1 and NOD2 signalling (Rip2-/-) and their heterozygote littermates (Rip2+/-). In Rip2-/- and Rip2+/- mice , ethanol administration of MDP significantly increased total colonic DCs, with Rip2+/- mice exhibiting a trend toward CD103+ cDC1 recruitment, and Rip2-/- mice skewed towards cDC2 recruitment. Finally, intestinal Treg compositions were compared, and as expected, only Rip2 sufficient animals displayed a notable increase in Tregs. This indicates that NOD1 and NOD2 signalling are crucial for the maintenance of cDC1 following gut permeability changes, and loss of this balance may shift the gut to a more inflammatory microenvironment.
Overall, this study provides a new perspective into the mechanism by which detection of MDP by NOD1 and NOD2 leads to the generation of Treg-promoting DCs and how dysregulation of this tolerogenic response may lead to Crohn’s disease. The finding that GM-CSF is important in the sensing of MDP by DCs opens up new avenues for therapy and prompts further research into this novel regulatory pathway, which could reveal promising therapeutic targets to tackle this devastating disease.
Primary Reference:
Prescott, D., Maisonneuve, C., Yadav, J., Rubino, S., Girardin, S., & Philpott, D. (2020). NOD2 modulates immune tolerance via the GM-CSF–dependent generation of CD103+ dendritic cells. Proceedings Of The National Academy Of Sciences, 117(20), 10946-10957.
Other references:
Impact of IBD in Canada Report – Resources and Publications – Crohn’s and Colitis Canada. Crohnsandcolitis.ca. (2020). Retrieved 4 November 2020, from https://crohnsandcolitis.ca/About-Us/Resources-Publications/Impact-of-IBD-Report.
Ogura, Y., Bonen, D., Inohara, N., Nicolae, D., Chen, F., & Ramos, R. et al. (2001). A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature, 411(6837), 603-606.
Ruane, D. T., & Lavelle, E. C. (2011). The role of CD103⁺ dendritic cells in the intestinal mucosal immune system. Frontiers in Immunology, 2, 25.
Leclerc, C., Bourgeois, E. & Chedid, L. (1982) Demonstration of muramyl dipeptide (MDP)-induced T suppressor cells responsible for MDP immunosuppressive activity. European Journal of Immunology, 12, 249–252.
Kishimoto, T., et al. (1979) Regulation of antibody response in different immunoglobulin classes. VI. Selective suppression of IgE response by administration of antigen-conjugated muramylpeptides. Journal of Immunology, 123, 2709–2715.
Coombes, J. L., Siddiqui, K. R., Arancibia-Cárcamo, C. V., Hall, J., Sun, C. M., Belkaid, Y., & Powrie, F. (2007). A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. Journal of Experimental Medicine, 204(8), 1757–1764.
Sun, C. M., Hall, J. A., Blank, R. B., Bouladoux, N., Oukka, M., Mora, J. R., & Belkaid, Y. (2007). Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. Journal of experimental medicine, 204(8), 1775–1785.
Collin, M. & Bigley, V. (2018). Human dendritic cell subsets: an update. Immunology, 154(1), 3–20.
Qiu, C. H., Miyake, Y., Kaise, H., Kitamura, H., Ohara, O., & Tanaka, M. (2009). Novel subset of CD8alpha+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. Journal of immunology, 182(7), 4127–4136.
Zhan, Y., Carrington, E. M., van Nieuwenhuijze, A., Bedoui, S., Seah, S., Xu, Y., Wang, N., Mintern, J. D., Villadangos, J. A., Wicks, I. P. & Lew, A. M. (2011). GM-CSF increases cross-presentation and CD103 expression by mouse CD8⁺ spleen dendritic cells. European journal of immunology, 41(9), 2585–2595.

Posted on : 29/07/2020 9:00 AM
Liked this post?
Click the social media icons on the left to share!
Have anything to say? Comment below!



